Inert Electrodes List
Electroanalytical potentiometry voltammetry polarography electroanalysis inert electrodes Inert electrodes active electrolysis chemistry Characteristics incorporated
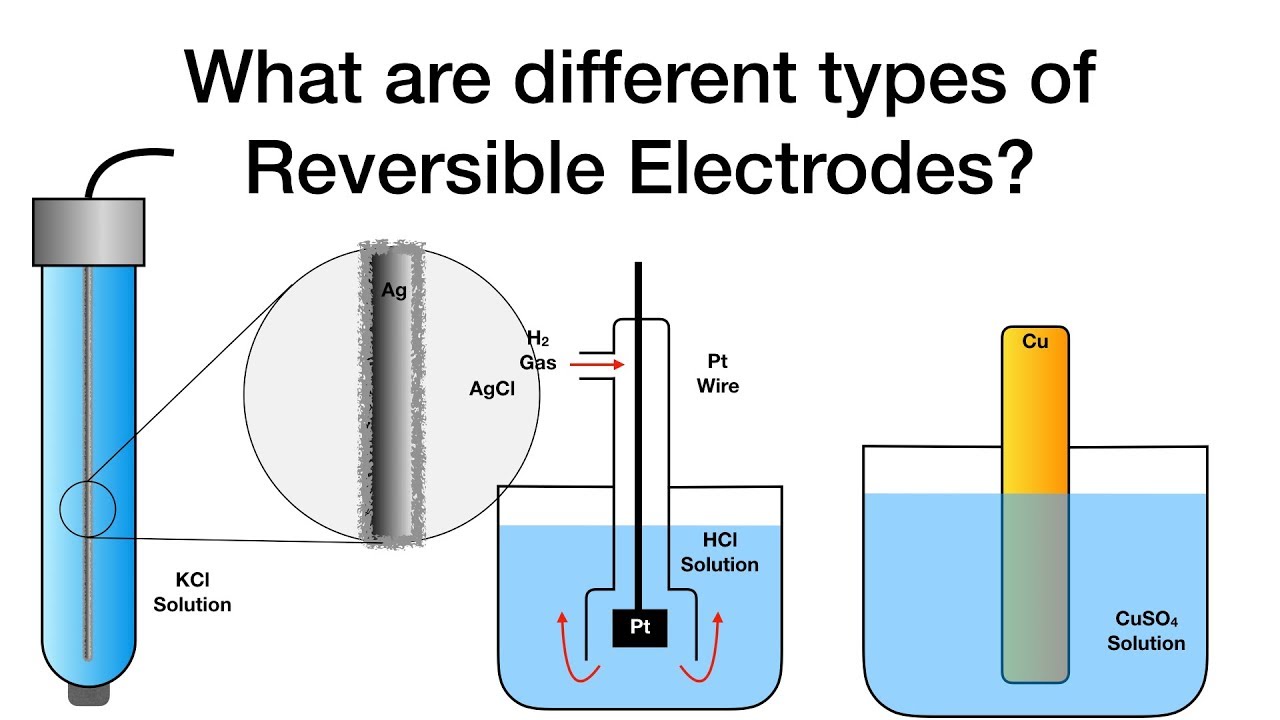
What are different types of Reversible Electrodes? | Electrochemistry
Mettler toledo electrode selective ion electrodes combination ise comb epoxid kefo fishersci Characteristics of the modified electrodes incorporated with different Electrochemical cell conventions
Electrode capacitance
Electrolysis of dilute sodium chloride (inert electrodes)Inert electrodes lets stage set ppt powerpoint presentation voltaic cell Inert electrodes voltaic cellsFactors affecting electrolysis.
Electrolysis electrodes inert ions appropriateWelding electrode e7018 electrodes smaw arc means meaning hydrogen understanding symbols low shielded following shows metal ndt example Mettler toledo ion selective electrodes (ise):ph and electrochemistryWelding electrodes – understanding the smaw electrode symbols.

Dilute electrolysis sodium chloride electrodes inert
Electrolysis electrodes inert reactive cuso4What are different types of reversible electrodes? Inert electrochemistry ppt powerpoint presentation anodeClassification of electrode.
Electrode inert electrodes typesTypes of electrodes with details of the component materials Aws classification electrode e7018 engineer diary example let4 inert electrodes.

Electroanalytical chemistry potentiometry voltammetry and polarography
Electrochemical electrode inert anode conventions cathode fe voltaic libretexts cellsComparison of various materials (electrode) according to their specific Factors affecting electrolysisElectrodes component materials.
Electrodes chemistry electrochemistry reversible types different physicalCh. 20-3 inert electrodes/voltaic cells Difference between active and inert electrodesInert electrolysis factors affecting electrodes platinum.

Inert electrodes electrolysis active difference between copper chloride natural diagram electrode graphite example vs setup grade atoms sciences figure
Electrode materials and propertiesElectrolysis affecting inert electrodes .
.